STEMSOFT FOR
The toolkit you need to electronically document and analyze your Immune Effector Cells (IEC) and CAR-T work including:
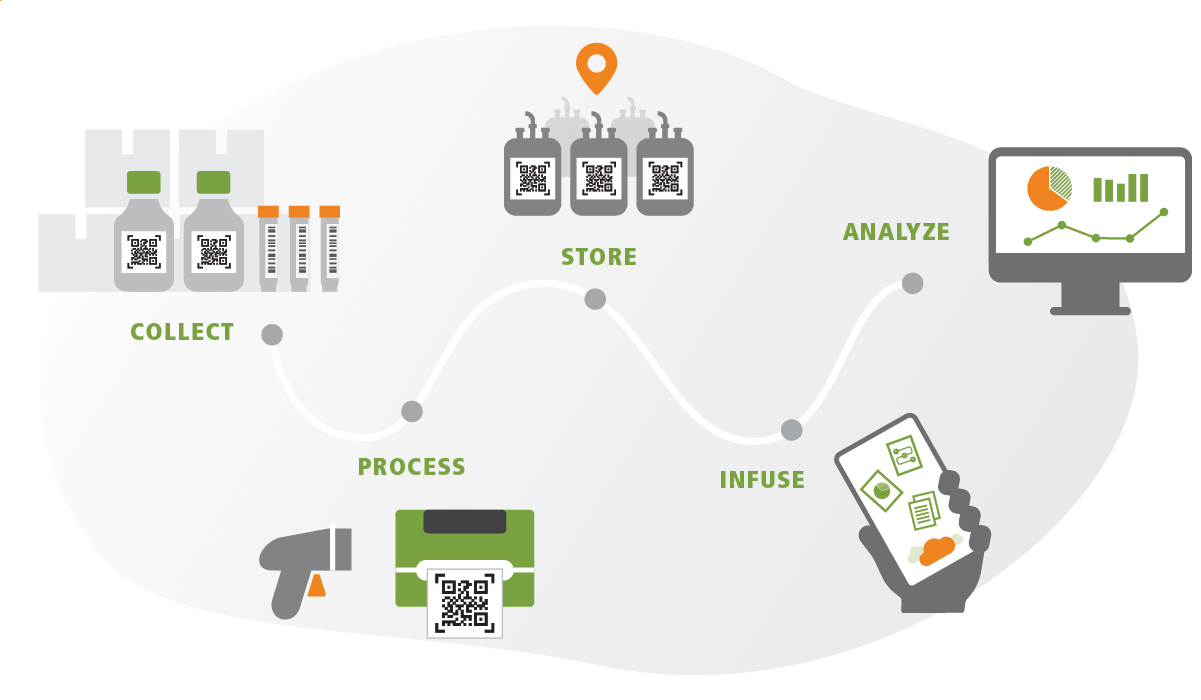
- Patient and consent tracking
- Apheresis collection, traceability and ISBT-128 labeling including the ISBT 128 Full Product for Cellular Therapy Manufacturing (CT) label
- Sponsor information
- Materials management (both internal and external)
- Chain of custody
- Chain of identity
- Processing and manipulations, including rescue cryopreservation of excess cells
- Shipment and logistics to Contract Manufacturing Organization (CMO)
- Receipt, quarantine storage and release of the CAR-T product from the CMO
- 21 CFR Part 11 and Annex 11 compliant electronic signatures
- Clinical outcomes and engraftment
- Adverse events, adverse reactions and Occurrences
- Analytics and data visualizations
STEMSOFT eBPR software helps you reduce redundant data entry, slips, lapses, and mistakes while also making it easier to maintain complete and accurate manufacturing records. Our software can help you maintain your FACT/JACIE and AABB accreditation and ensures compliance with cGMP, cGTP, GDPR, HIPAA and more.
Our proprietary Jump Start Implementation Program can help your team get up and running quickly with a proven solution including:
- Standardized, scheduled training with presentation materials, case studies, homework and quizzes to reinforce the live sessions
- Access to a training site to practice at your pace
- Access to industry standard workflows
- Sample process documents to help organize your laboratory processes
- Sample Standard Operating Procedures
- Related supporting documentation for accreditation and regulatory compliance
Book your demonstration today and learn how STEMSOFT will help you take control of your CAR-T processes.
Ready to learn more?
Software Solutions to Support Your Cell Therapy Workflow
Your Software Solution
STEMSOFT LAB
Track all of your unique operational and manufacturing details in one platform, with built-in support for accreditation compliance, processing standardization and data analysis.
Receive Extended Support with Your STEMSOFT Solution
At STEMSOFT, we know that the proper software solution is only one piece of your cell therapy program. That’s why we’re proud to offer additional services and resources to help you get the most out of your STEMSOFT system.
Support Portal
As a valued client, you’ll gain access to our users-only Support Portal offering extensive supporting documentation, example Standard Operating Procedures (SOPs), a searchable knowledgebase, video library, newsletters, and more.
Software Implementation and Ongoing Support
With over 25 years of experience in implementing cellular therapy software solutions, we have developed a structured training approach that will enable your team to easily get started with STEMSOFT today while also providing you with the ongoing training, upgrades, and support you need for the long term.


