STEMSOFT FOR
Translating novel products from bench to bedside is a unique challenge. Translational products can be a hybrid of novel and conventional processes, with evolving requirements as you refine and elaborate the manufacturing process. STEMSOFT batch record software is highly configurable, with an implementation methodology that allows you to focus on novel processes, adapting your documentation strategy to match your level of manufacturing and regulatory maturity.
STEMSOFT commercial-off-the-shelf batch record and manufacturing software is configurable and workflow-driven. Integrated support for barcoding, instrument integration, in-process calculations, and business intelligence ensure that you have accurate and complete records, and the ability to mine them for insights in real time. STEMSOFT products are continuously updated in line with the ever-changing regulatory and accreditation landscape affecting the cellular therapy community.
STEMSOFT eBPR software helps you reduce duplicate data entry, slips, lapses, and mistakes while also making it easier to maintain complete and accurate manufacturing records. Our software can help you maintain your FACT/JACIE and AABB accreditation; ensure compliance with cGMP, cGTP, GDPR, HIPAA, 21CFR Part 11, Annex 11 and ISBT; and generate the data you need to ensure that your team consistently delivers standard of care and advanced medicinal products.
Ready to learn more?
Software Solutions to Support Your Entire Translational Medicine Workflow
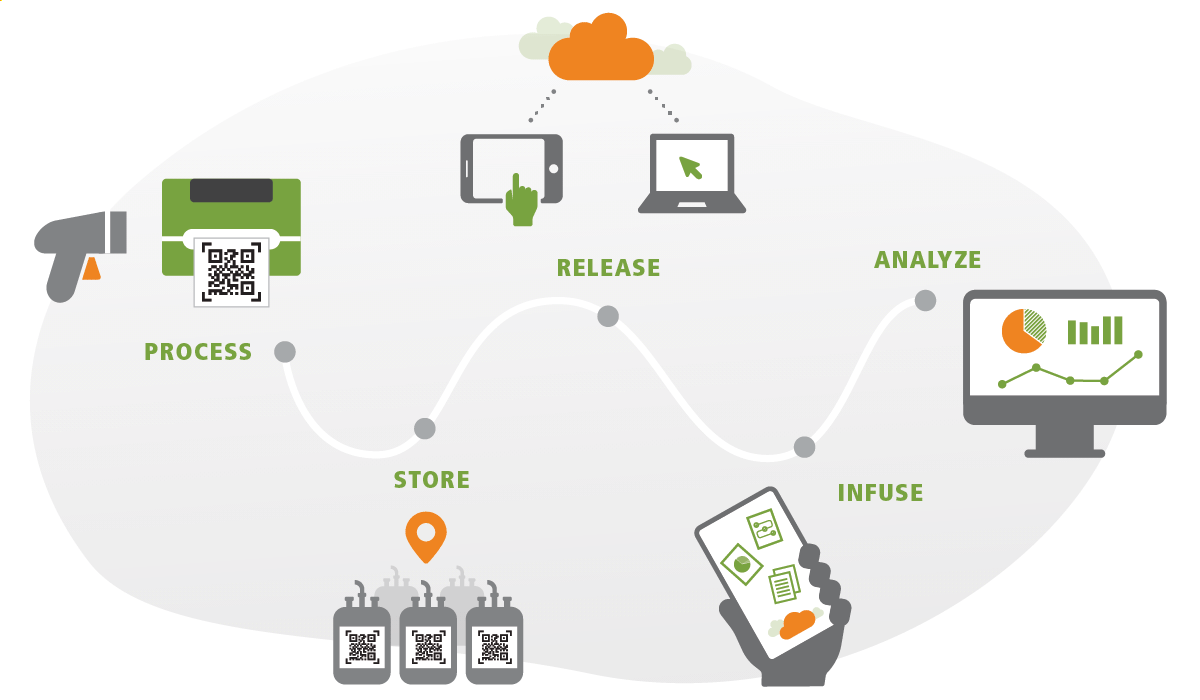
Your Software Solution
STEMSOFT LAB
Track all of your unique operational and manufacturing details in one platform, with built-in support for accreditation compliance, processing standardization and data analysis.
Receive Extended Support with Your STEMSOFT Solution
At STEMSOFT, we know that the proper software solution is only one piece of your cell therapy program. That’s why we’re proud to offer additional services and resources to help you get the most out of your STEMSOFT system.
Support Portal
As a valued client, you’ll gain access to our users-only Support Portal offering extensive supporting documentation, example Standard Operating Procedures (SOPs), a searchable knowledgebase, video library, newsletters, and more.
Software Implementation and Ongoing Support
With over 25 years of experience in implementing cellular therapy software solutions, we have developed a structured training approach that will enable your team to easily get started with STEMSOFT today while also providing you with the ongoing training, upgrades, and support you need for the long term.


